

There you can find the metals, semi-conductor(s), non-metal(s), inert noble gas(ses), Halogens, Lanthanoides, Actinoids (rare earth elements) and transition metals.Ĭlick here: for a schematic overview of the periodic table of elements in chart form Please note that the elements do not show their natural relation towards each other as in the Periodic system. Q: Arrange these elements according to electronegativity.
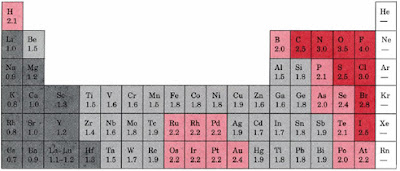
The first chemical element is Cesium and the last one is Helium. The chemical elements ofįor chemistry students and teachers: The tabular chart on the right is arranged by Ionization energy. Figure 3.2.2 Definitions of the Atomic Radius. This list contains the 118 elements of chemistry. Atomic radii are often measured in angstroms (Å), a non-SI unit: 1 Å 1 × 1010 m 100 pm. Phone: +31 152 610 900 of the innovation award 2017Ĭhemical elements listed by ionization energy The elements of the periodic table sorted by ionization energyĬlick on any element's name for further information on chemical properties, environmental data or health effects. ELECTRONEGATIVITY: 1: H: Hydrogen: 2.20: 2: He: Helium: no data: 3: Li: Lithium: 0.98: 4: Be: Beryllium: 1.57: 5: B: Boron: 2.04: 6: C: Carbon: 2.55: 7: N: Nitrogen: 3.04: 8: O: Oxygen: 3.44: 9: F: Fluorine: 3.98: 10: Ne: Neon: no data: 11: Na: Sodium: 0.93: 12: Mg: Magnesium: 1.31: 13: Al: Aluminum: 1.61: 14: Si: Silicon: 1.90: 15: P.Plant Inspection & Process Optimalisation.If you remember that fact, everything becomes easy, because electronegativity must always increase towards fluorine in the Periodic Table. The most electronegative element is fluorine. Since the electronegativity of some of the important elements cannot be determined by these trends (they lie in the wrong diagonal), we have to memorize the following order of electronegativity for some of these common elements.į > O > Cl > N > Br > I > S > C > H > metals The overall trend for electronegativity in the periodic table is diagonal from the lower left corner to the upper right corner. Elements at the top of a column have greater electronegativities than elements at the bottom of a given column. The result of this change is that electronegativity increases from bottom to top in a column in the periodic table even though there are more protons in the elements at the bottom of the column. Most electronegative N o Na Al Cs Least electronegative. It is readily seen from these numbers that, as the distance between the charges increases, the force decreases very rapidly. Question: Arrange these elements according to electronegativity. In this expression, Q represents a charge, k represents a constant and r is the distance between the charges. The force between two charges is given by Coulomb’s law. The distance of the electrons from the nucleus remains relatively constant in a periodic table row, but not in a periodic table column. Patterns of electronegativity in the Periodic Table The bond is then an ionic bond rather than a covalent bond. To all intents and purposes, A has lost control of its electron, and B has complete control over both electrons. If B is a lot more electronegative than A, then the electron pair is dragged right over to B's end of the bond. The hydrogen-chlorine bond in HCl or the hydrogen-oxygen bonds in water are typical. In the diagram, "\(\delta\)" (read as "delta") means "slightly" - so \(\delta+\) means "slightly positive".Ī polar bond is a covalent bond in which there is a separation of charge between one end and the other - in other words in which one end is slightly positive and the other slightly negative. C < N < O < F Electronegativity decreases as you move down a group in the. Solution: A Electronegativity increases from lower left to upper right in the periodic table (Figure 8.4.2). Classify each element as a metal, a nonmetal, or a metalloid according to its location about the diagonal belt of metalloids running from B to At. At the same time, the A end (rather short of electrons) becomes slightly positive. Electronegativity increases left to right across a row in the periodic table e.g. Arrange the elements in order of increasing electronegativity. That means that the B end of the bond has more than its fair share of electron density and so becomes slightly negative.
What if B is slightly more electronegative than A?ī will attract the electron pair rather more than A does.


 0 kommentar(er)
0 kommentar(er)
